Current research projects and topics of interest
The SYMBIOSE Lab investigates the physical principles governing biological systems
Our research focuses on changes in the physico-chemical properties of the extracellular matrix such as how curvature, viscoelasticity and spatial confinement influence cell behavior, with particular emphasis on cell migration and nuclear mechanics.
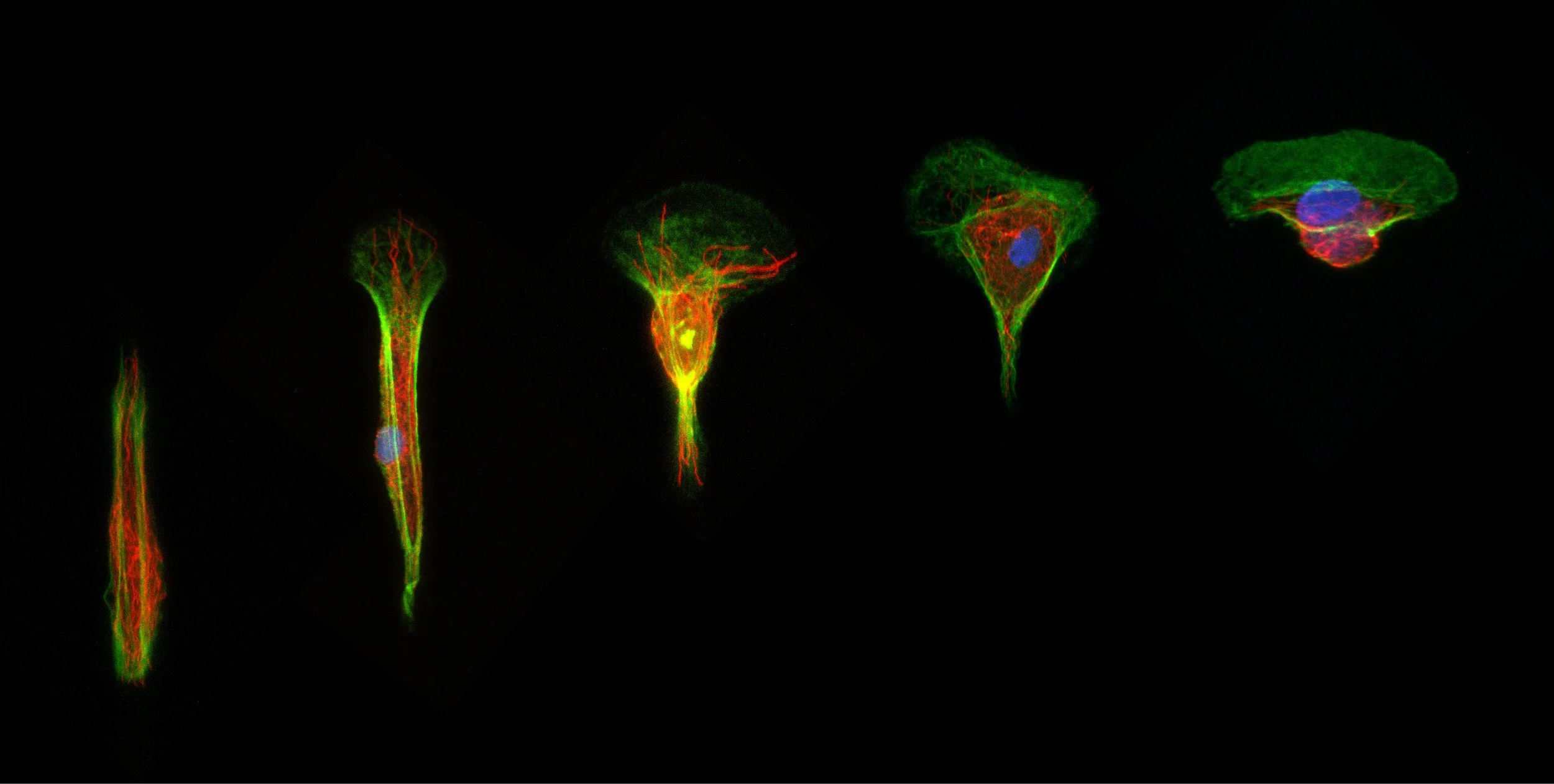
Epithelia are not flat but generally curved. To understand how variations in positive and negative curvature can modulate the mechanical balance of epithelial tissues and regulate their functions, we developed a photopolymerization method that allows us to mimic complex curvatures while simultaneously controlling matrix stiffness. Using a combined experimental and theoretical approach—based on vertex models—we formulated the hypothesis that cells may sense curvature by modulating tissue thickness. We found that local curvature also influences nuclear morphology and positioning. This phenomenon was explained by extending the vertex model to include membrane–nucleus interactions, thereby linking curvature-induced changes in tissue thickness to nuclear deformation and displacement.
R.K. Sadhu et al. Proc. Natl. Acad. Sci. USA 121, e2306818121 (2024) M. Luciano et al. Nature Reviews Physics 6, 246-268 (2024) M. Luciano et al. Advanced Healthcare Materials 13, 2203377 (2024) M. Luciano et al. Nature Physics 17, 1382-1390 (2021)
Matrix curvature
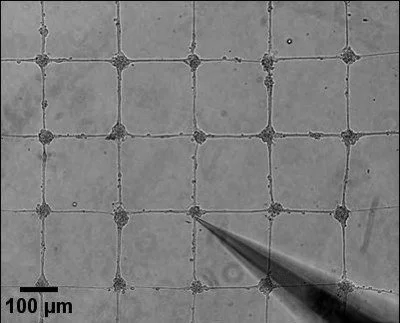
We study the collective behavior of epithelial cells in terms of cell polarity and migration. To this aim, we develop microfabricated tools and microforce assays to control the physico-chemical environment of cell assemblies. By combining these tools with advanced imaging techniques, we investigate how the physical properties of the cell microenvironment modulate collective cell migration. We use well-designed cell clusters to decipher the role of cell-cell junctions and how physical constraints, such as the spatial confinement, can lead to emergent dynamical and mechanical properties of epithelial tissues.
Y. Kalukula et al. Nature Physics in press (2025) E. Vercruysse et al. Nature Physics 20, 1492–1500 (2024) M. Versaevel et al. Scientific Reports 11, 1-11 (2021) D. Mohammed et al. Nature Physics 15, 858-866 (2019)
M. Riaz et al. Scientific Reports 6, 1-14 (2016)
Single and collective cell migration
Time-lapse sequence of the back and forth motion of a single epithelial cell (MCF-10A) migrating on a dumbbell-shaped micropattern composed of a thin bridge of 6 µm wide and 160 µm long connected to deconfinement squares of 40x40 µm.
Breast tissues: confined migration, viscoelasticity and mechanical memory
Cancer metastasis relies on cell migration and dissemination, processes that are closely associated with physico-chemical changes in extracellular matrix (ECM) properties and spatial confinement. Tumor cells must navigate through narrow interstitial spaces to invade distant organs. Using microfabricated devices and novel viscoelastic hydrogels, we aim to elucidate how matrix viscoelasticity and dimensionality interact to drive tumor progression. We are particularly interested in both the short- and long-term consequences of mechanical stress on the cytoskeleton and nucleus, including chromatin (re)organization and genomic instability. Ultimately, this work aims to provide new insights into the mechanisms of cell dissemination in breast tissue, to uncover a mechanical memory mechanism in confined migrating cells and to identify novel therapeutic targets for preventing metastasis, particularly in breast cancer.
Y. Kalukula et al. Nature Physics in press (2025) G. Ciccone et al. Advanced Science 12, 2408635 (2025)
Neuronal and glial cell mechanics
We develop an interdisciplinary approach to investigate how cellular forces, local cell and tissue mechanical properties contribute to the modulation of neuronal and glial functions. By using cell stretcher, micropatterned substrates, complex compliant cell culture substrates and confocal laser scanning microscopy, we study the mechanical activation of brain cells (neurons, astrocytes and glial cells) and their interactions. We aim to understand how neurons and glial cells respond to external forces and mechanical cues of their microenvironment. Our long-term goal is to elucidate the molecular mechanisms of traumatic brain injury (TBI) and to participate to the development of effective therapeutic solutions.
A. Procès Neural Regeneration Research 20, 2304-2306 (2025) A. Procès et al. Biomaterials 305, 122426 (2024). J. Lantoine et al. ACS Chemical Neuroscience 12, 3885-3897 (2021)
J. Lantoine et al. Biomaterials 89, 14-24 (2016)
T. Grevesse et al. Scientific Reports 5, 1-10 (2015)
We develop biomaterial scaffolds with highly-controlled architectures and chemical functionalization for two- and three-dimensional cell culture, tissue regeneration, and biological assays. We aim to understand how cells and tissues actively interact with their surroundings to sense, store and exchange information by capturing key features of the biochemical and biophysical aspects of a cell’s niche. Our approach combines the principle of soft lithographic processes with photopolymerized polymers to engineer biomaterial niches as physiologically or pathologically-relevant models, while mimicking the physico-chemical properties of the cell’s niche.
M. Luciano et al. Soft Matter 21, 4551-4572 (2025) M. Luciano et al. Advanced Healthcare Materials 13, 2203377 (2024) M. Luciano et al. Nature Physics 17, 1382-1390 (2021)
J. Lantoine et al. Biomaterials 89, 14-24 (2016)
Bioengineered hydrogels
Current collaborative research projects
Our lab is participating in two european research (MICROPLAITE and AntiResi) projects funded by the Interreg VI Programme
which is a European territorial cooperation programme Interreg France-Wallonie-Vlaanderen that aims to foster cross-border exchanges between the Hauts-de-France and Grand Est regions, Wallonia, and both West and East Flanders.
The rising prevalence of chronic diseases, cancer, burns, and diabetes is creating a growing need for innovative solutions in tissue reconstruction, particularly to address soft tissue defects associated with these conditions. The aim of the MICROPLAITE project is to develop an innovative microfluidic platform designed to accelerate the development of medical devices for tissue reconstruction.
The aim of the AntiResi project is to proactively address the growing issue of antibiotic resistance by developing innovative technological solutions for three types of materials to combat bacterial infections and the emergence of persistent forms.